
The details of ionic bonds can seem confusing. The ionic bond definition is a chemical bond formed when one atom gives up one or more electrons to another atom. ExploreLearning Learning Designer Lauren Schetne, Ph.D., breaks down the answer to this question: How does ionic bonding work?
What is an ionic bond?
“Ionic bonds happen between atoms with differing electronegativity. This refers to how well an atom can hold onto its electrons,” Schetne said. “An atom with weak electronegativity cannot hold on to its electrons very well, versus an atom with high electronegativity does.” Atoms are arranged in the periodic table according to their electronegativity. Ionic bonds occur between atoms with opposite electronegativities.
.webp)
Ionic vs. covalent bonds: Key differences
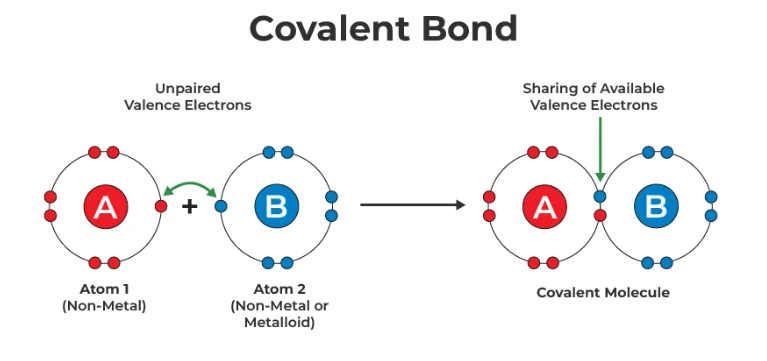
There are a few differences between ionic and covalent bonds. Let’s start with one key difference. In ionic bonds, one atom transfers electrons to another, creating an oppositely charged ion. Atoms share electrons to form a bond in covalent bonds. Another example involves metals and nonmetals. Ionic bonds usually occur between the two, while covalent bonds occur between nonmetals.
Schetne noted, “In a covalent bond, electrons are not donated or stripped from atoms but are instead shared between atoms. And covalent bonds are much stronger than ionic bonds.”
How do you explain ionic bonds to students?
When complicated information is broken down into bite-sized chunks, the concepts are more digestible – or understandable. This is a good approach for how to teach ionic bonding. Start with atoms. “All atoms have shells of energy called electron shells. The outermost shell is called a valence shell,” said Schetne. “Atoms are ‘happiest’ when they have a valence shell that is full – in other words, it contains the maximum number of electrons that valence shell can hold.”
When atoms have incomplete shells, they will either donate or accept electrons to fill their shell. Schetne explained further with this example:
Take NaCl or table salt – Sodium (Na) only has one electron in its valence shell, which can hold a maximum of 8 electrons. Chlorine (Cl) has a valence shell with 7 electrons (1 empty space). So, when these atoms come close together, sodium will donate one electron to chlorine.
This donation causes sodium to become positively charged, and chlorine is now negatively charged. Because these atoms now have charges, they are referred to as ions.
These opposite charges are attracted to each other and form an ionic bond.
.webp)
Common misconceptions about ionic bonds and how to address them
Misconceptions happen in the learning process. A common one involving ionic bonds is that they share electrons between atoms. The truth is that ionic bonds form when one atom completely transfers electrons to another, which creates oppositely charged ions that attract each other.
Another misconception is thinking that ionic bonds are always 100% ionic (most bonds have some degree of covalent character), believing that a single sodium atom is directly bonded to a single chlorine atom in sodium chloride, and not understanding that the attraction between the ions is what constitutes the bond, not the electron transfer itself. What about polarity in ionic bonds? Some think there is no polarity, but that doesn’t always prove true.
To address the misconceptions, keep the discussion open. Create an atmosphere where students feel comfortable making predictions and explaining their thinking. Reteach with new examples—ask other students to use their own words to explain to the class. Tables and graphic organizers help visualize complex concepts. And, of course, frequently check for understanding.
Using virtual labs to teach ionic bonding
Virtual labs, like ExploreLearning Gizmos, help students access and understand big STEM ideas because they encourage scientific thinking and nurture natural curiosity. They give students opportunities to investigate and develop good scientific models. Virtual labs teach students to use evidence as they develop a deeper understanding, come up with new questions, and revise their models. Through these processes, students can visualize and understand complex systems just like scientists do in the real world.
Benefits of virtual labs in teaching ionic bonds
Students who use virtual labs can build a deeper conceptual understanding of STEM subjects. Virtual labs allow students to observe things that are otherwise unobservable. That’s true with ionic bonds. “Atoms cannot be visualized in the classroom. Using models or simulations can allow students to visualize subatomic particles in an easy way,” said Schetne.
Engaging students with Gizmos’ ionic bond labs
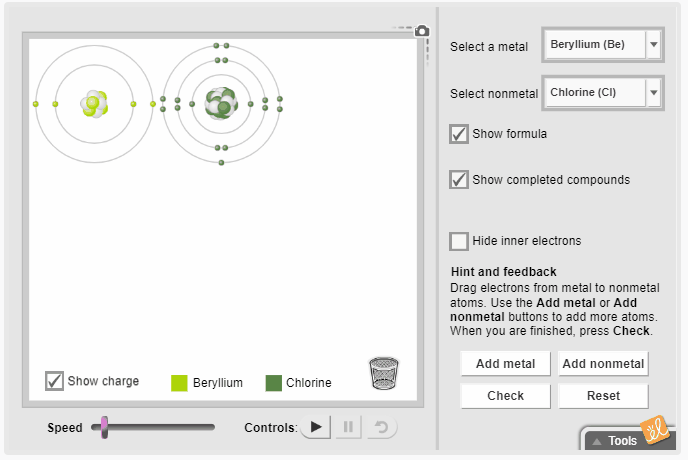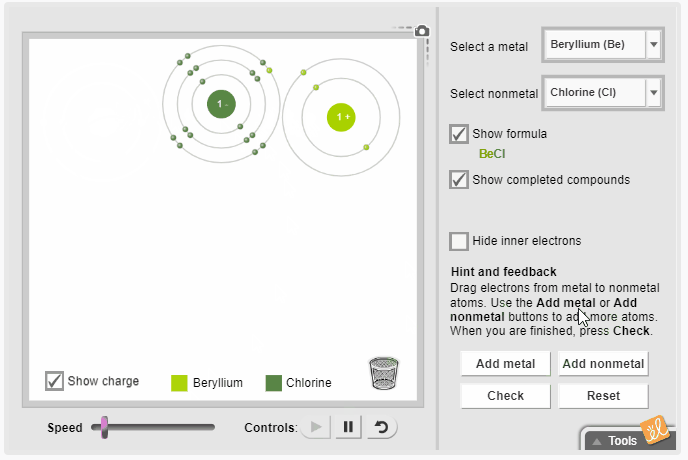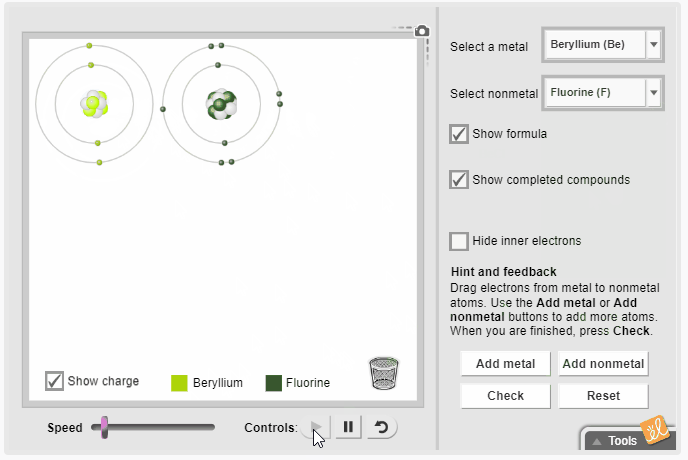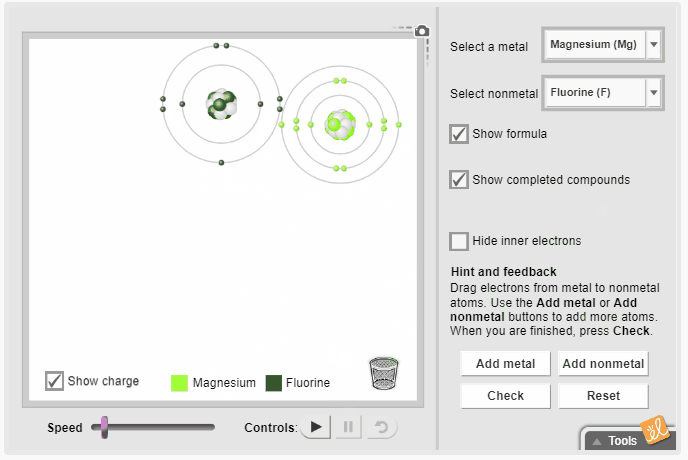
Gizmos allow students to visualize complex ideas, test their own theories, and even predict outcomes. The Ionic Bonds Gizmo simulates ionic bonds between a variety of metals and nonmetals. Students select a metal and a nonmetal atom and transfer electrons from one to the other. They observe the effect of gaining and losing electrons on charge and rearrange the atoms to represent the molecular structure. Additional metal and nonmetal atoms can be added to the screen, and the resulting chemical formula can be displayed.
Activities to reinforce ionic bonding concepts
Looking for more ways to reinforce ionic bonding concepts? Manipulatives and games add fun elements to reinforcement and remediation. Here are some activities to teach ionic bonding:
Matching Game: Students create cards representing different ions with their charges, then match pairs of ions with opposite charges to form stable ionic compounds.
Make Models: Ask students to represent atoms and their electrons with things like colored beads, magnetic tiles, or pipe cleaners. They can actually transfer electrons to create ions.
Role Playing: Divide students into pairs (one is a metal atom, and the other is a non-metal atom) to act out the process during electron transfer.
Real-Life Examples: Relate the complex concept to things students already know and use. Share some (table salt and toothpaste with fluoride), then have students research to make a class list.
Conclusion: Simplifying ionic bonds with virtual labs
Gizmos allow equitable access to learning experiences that simplify complex concepts. Teachers can deliver comprehensive lessons in efficient, research-based, and cost-effective ways. Students tackle the concept of ionic bonds with opportunities to gather data, analyze, infer, and discuss results along the way, making meaningful connections to solidify learning.
With a free Gizmos trial, you can help your students better understand ionic bonds and other complex STEM concepts.