
In 1789, French chemist Antoine Lavoisier worked to group the elements as metals and nonmetals. Almost a hundred years later, in 1869, a Russian chemist named Dmitri Mendeleev constructed a periodic table with gaps for elements that had yet to be discovered. That periodic table of horizontal rows and vertical columns is still used today.
Why is the periodic table sometimes challenging for students?
When students attempt to learn the periodic table, the amount of information might initially seem overwhelming. The rows and columns are key to explaining the periodic table. When teaching how to use the periodic table, students need to understand the way the information is organized.
- Learning the elements starts with learning the table itself. Horizontal rows, which are called periods, have metals on the left and nonmetals on the right.
- Vertical columns, also known as groups, have elements with similar chemical properties.
Why is the periodic table important?
Organization and structure are critical to understanding complex concepts. That’s why the periodic table is so important. It puts all the chemical elements in order of increasing atomic number or the total number of protons in the atomic nucleus. This creates a recurring pattern called the “periodic law,” with elements in the same column (group) having similar properties, which makes it easier for scientists to predict reactions between elements and make calculations.
How do you explain the periodic table to students?
As with any complex concept, the periodic table should be divided into manageable sections. When students understand the smaller parts and overall organization of the table, the bigger picture becomes clearer. Relating the table to real life is also important; otherwise, the elements could be too abstract for students to grasp.
Even though scientists and other STEM professionals use the periodic table, students may not realize how essential it is to daily life. For example, oxygen is necessary for respiration, circulation, and other body processes. Calcium helps to make stronger teeth and bones, and chlorine is used as a disinfectant.
Teaching strategies to make the periodic table fun
Learning the elements is perfect for creativity because explaining the periodic table relates to other subject areas! Don’t limit students to a single periodic table of elements activity. Offer a few periodic table activities for students to understand its importance and relevance to real life fully.
- Create a memory game version of the elements by putting the symbol on one side and the common name on the other.
- Research careers, like astrophysicists, chemists, and doctors, that use the periodic table of elements.
- Use art skills for designs and sketches to represent the elements.
- Play Element Bingo by making cards with a variety of elements and simple prizes for winners.
- Take a Scavenger Hunt around the classroom or school to find items that match elements on a list. Students could even find items and determine the elements themselves.
- Ask students to write about 5-10 elements they use every day with explanations of importance.
Using Gizmos to teach the periodic table
Gizmos virtual simulations deliver comprehensive lessons for explaining the periodic table. Gizmos make it easy to make the periodic table interesting, informative, and interactive. Take a look at these options.
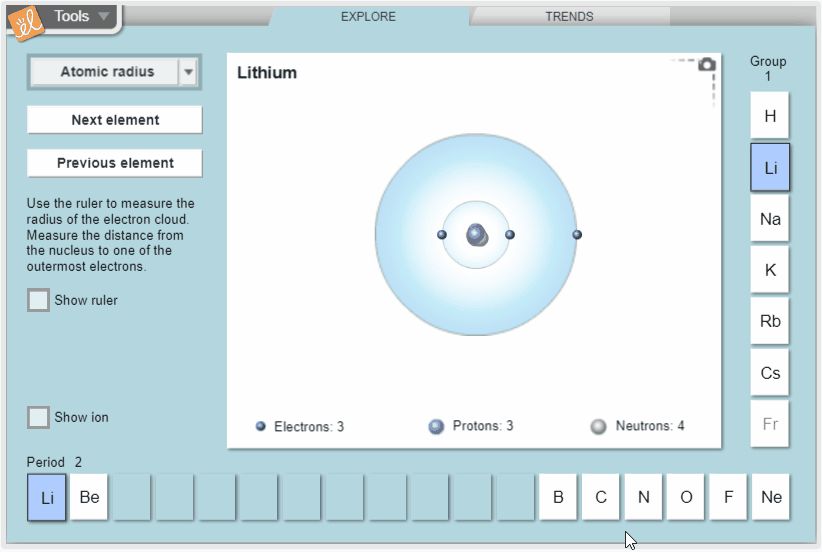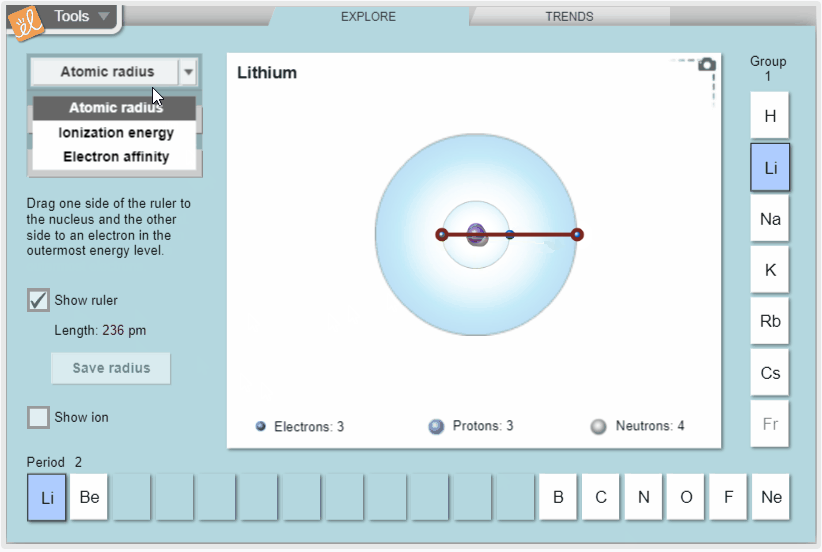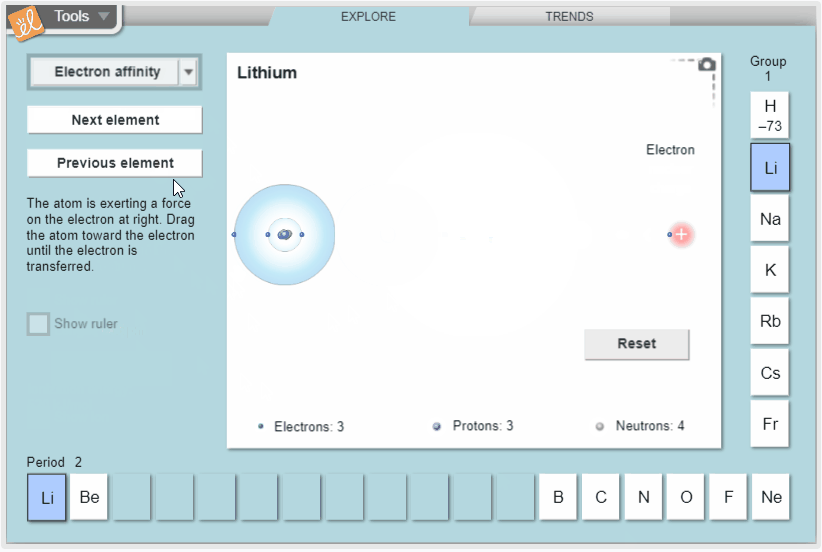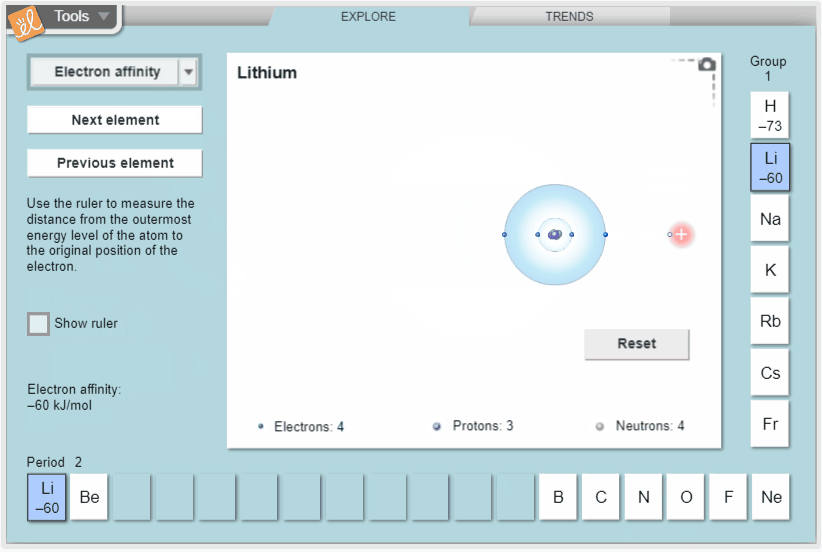
Explore trends in atomic radius, ionization energy, and electron affinity in the periodic table. Measure atomic radius with a ruler and model ionization energy and electron affinity by exploring how easy it is to remove electrons and how strongly atoms attract additional electrons. View these properties on the whole periodic table to see how they vary across periods and down groups.
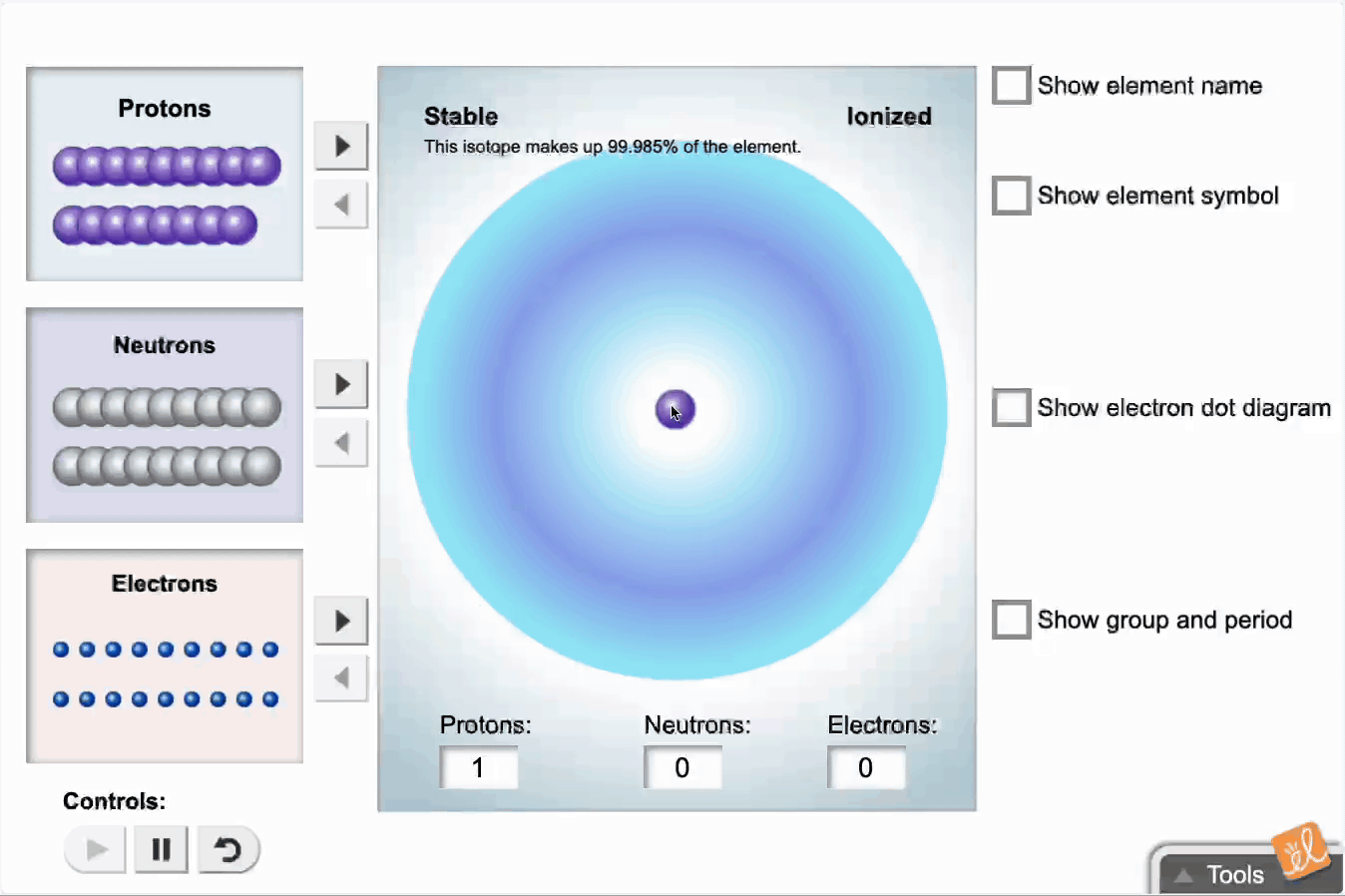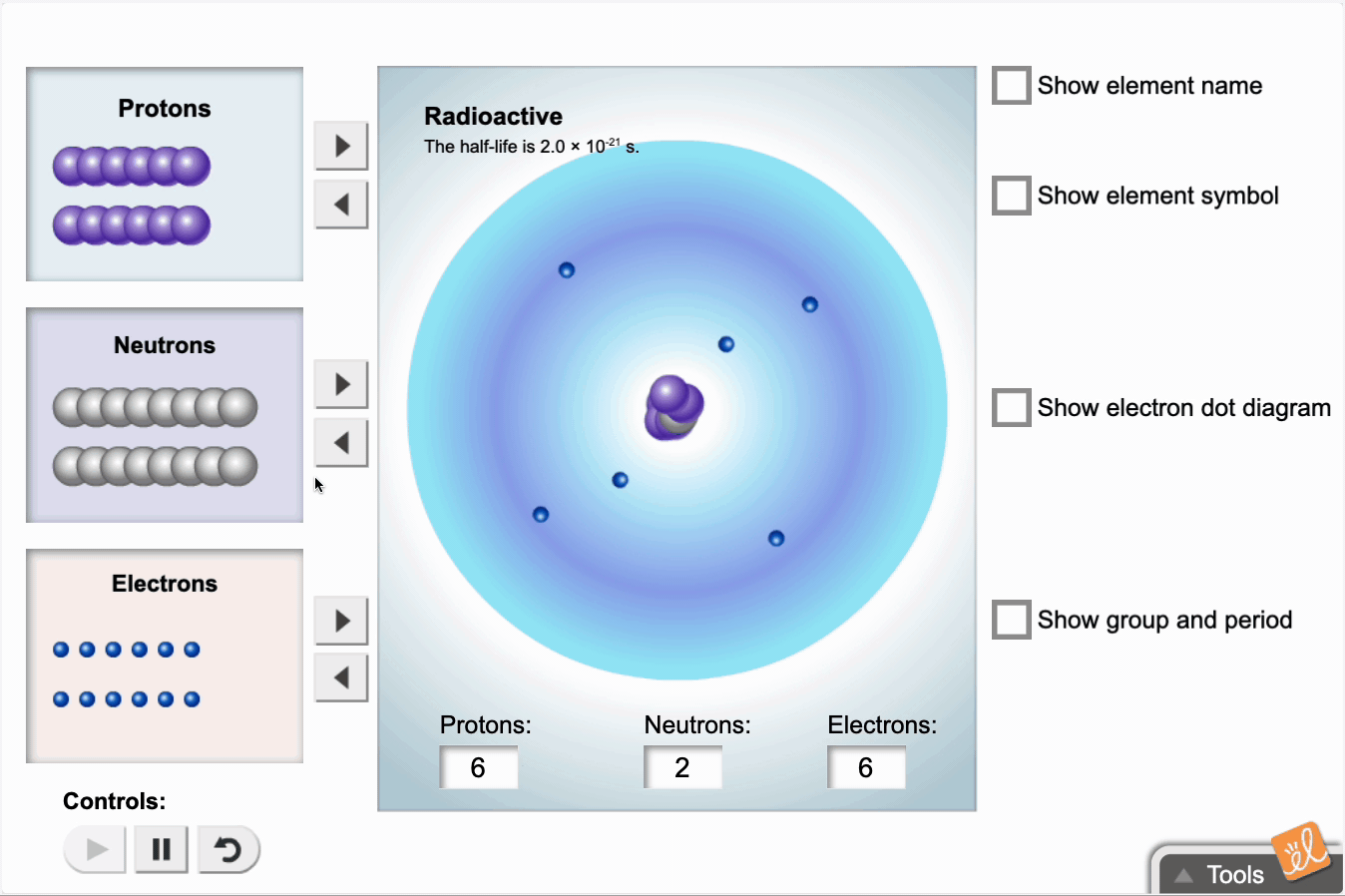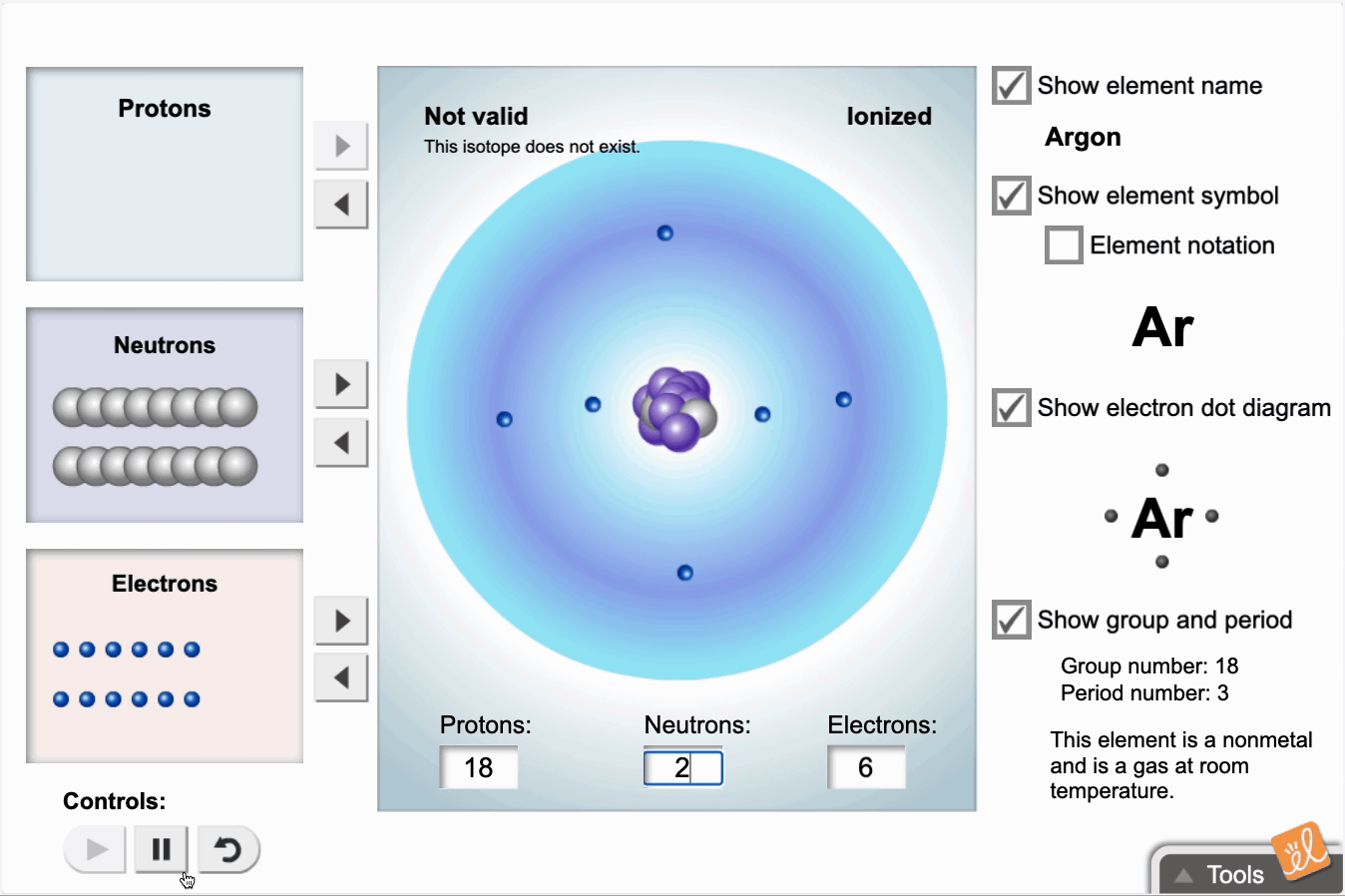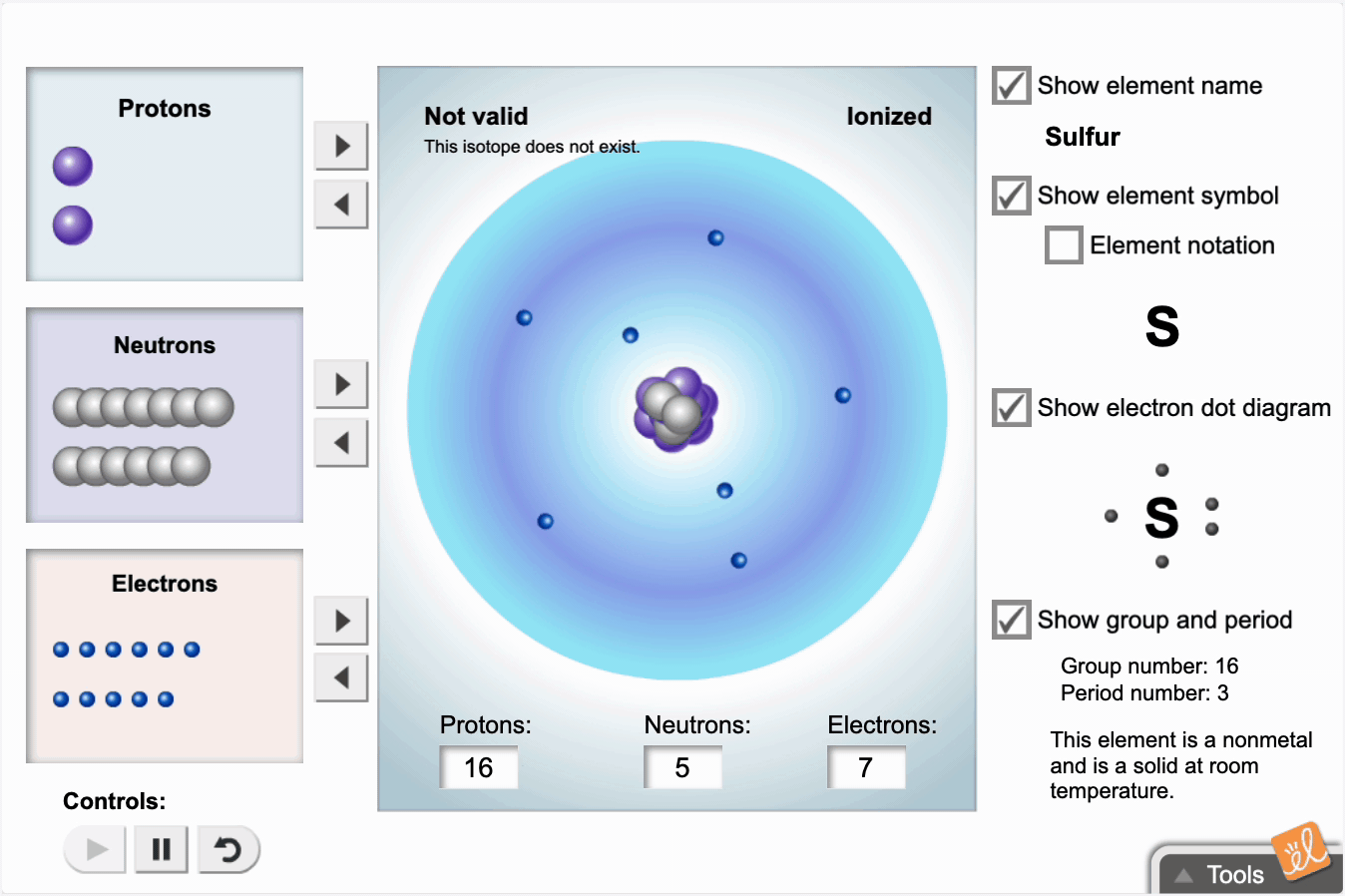
Use protons, neutrons, and electrons to build elements. As the number of protons, neutrons, and electrons changes, information such as the name and symbol of the element, the Z, N, and A numbers, the electron dot diagram, and the group and period from the periodic table are shown. Each element is classified as a metal, metalloid, or nonmetal, and its state at room temperature is also given.
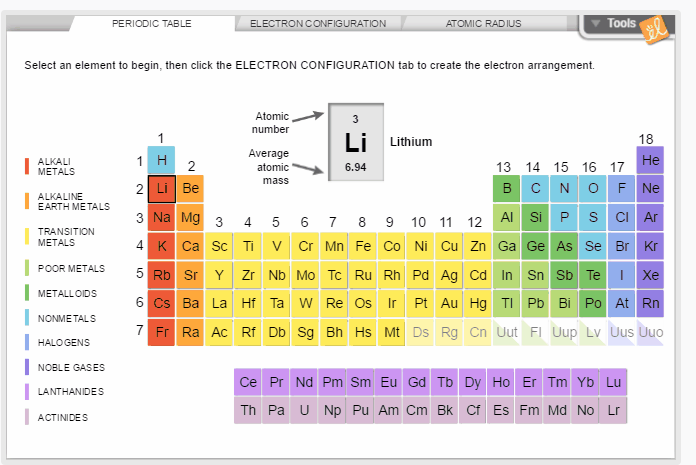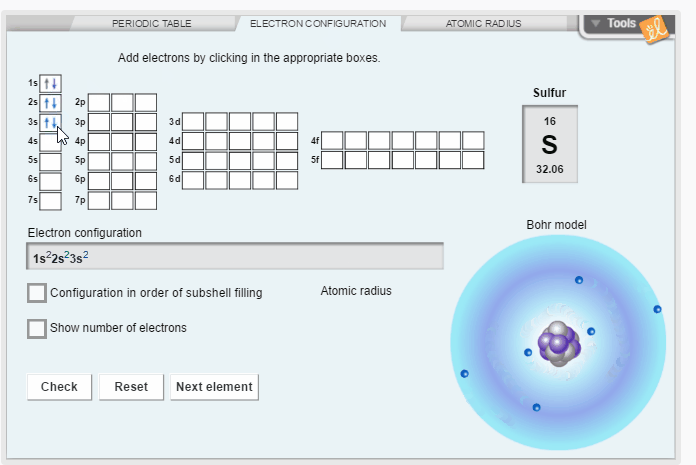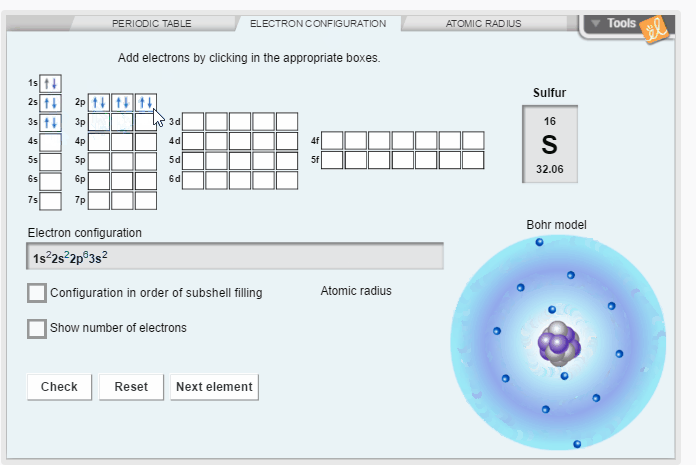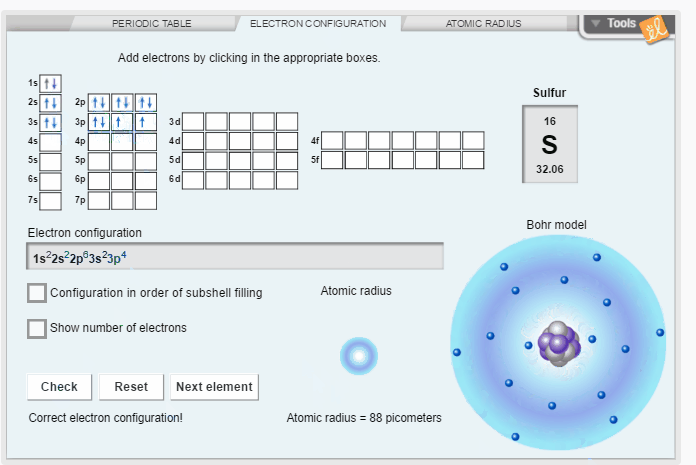
Create the electron configuration of any element by filling electron orbitals. Determine the relationship between electron configuration and atomic radius. Discover trends in atomic radii across periods and down families/groups of the periodic table.
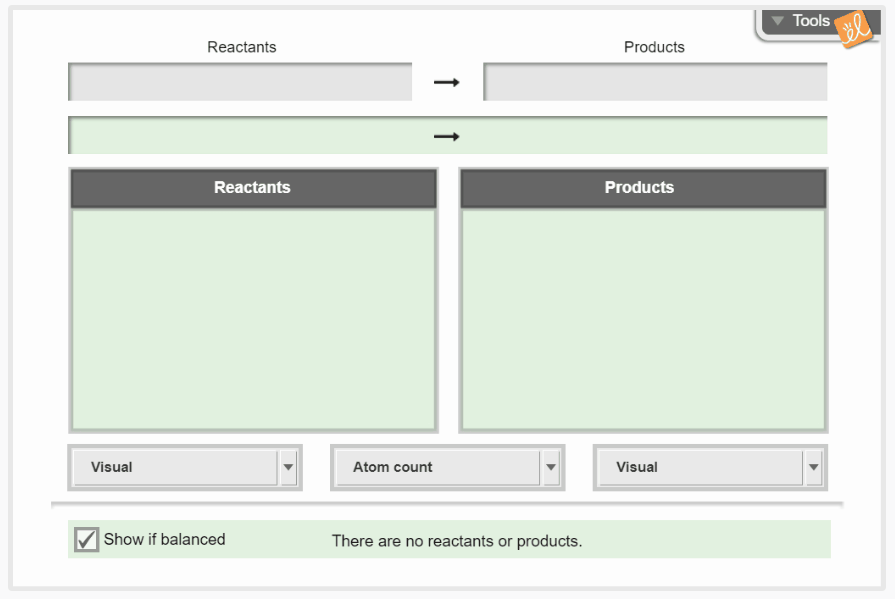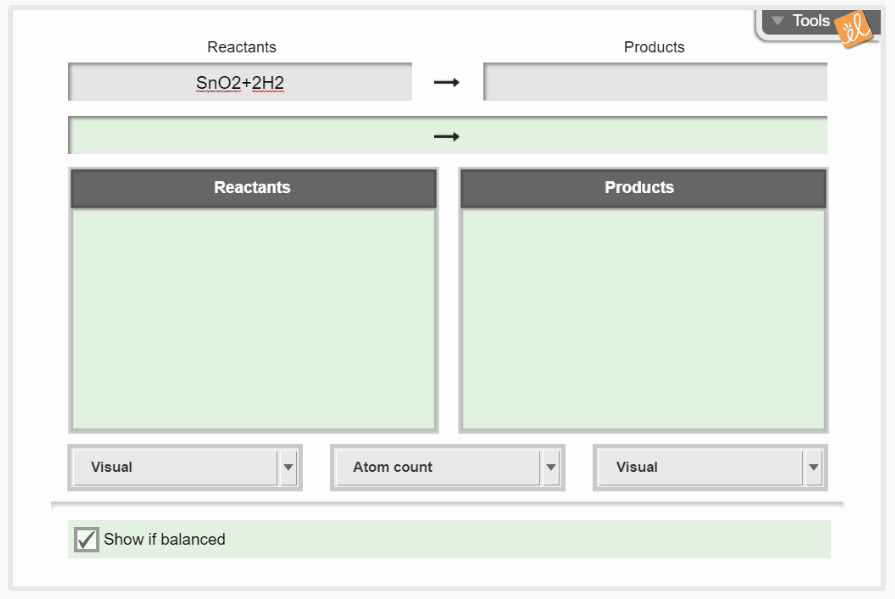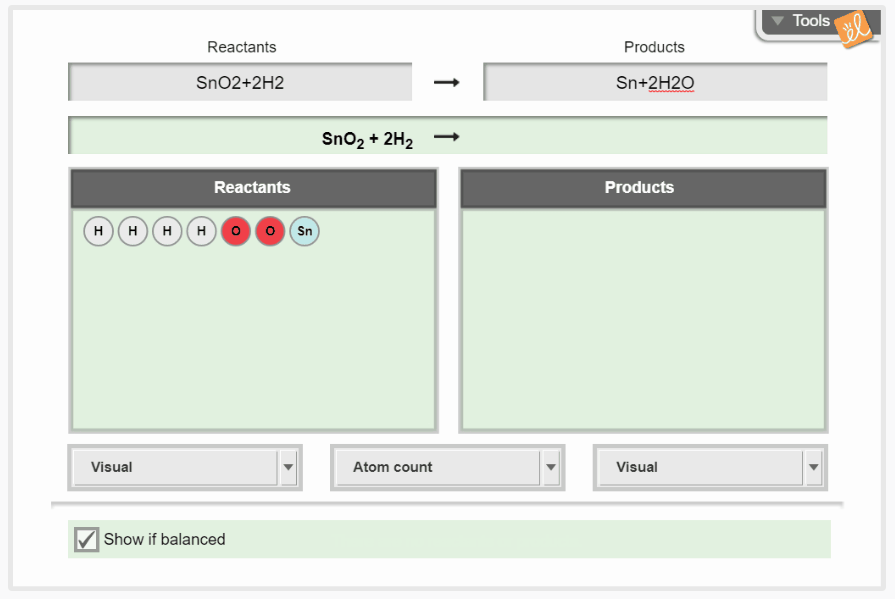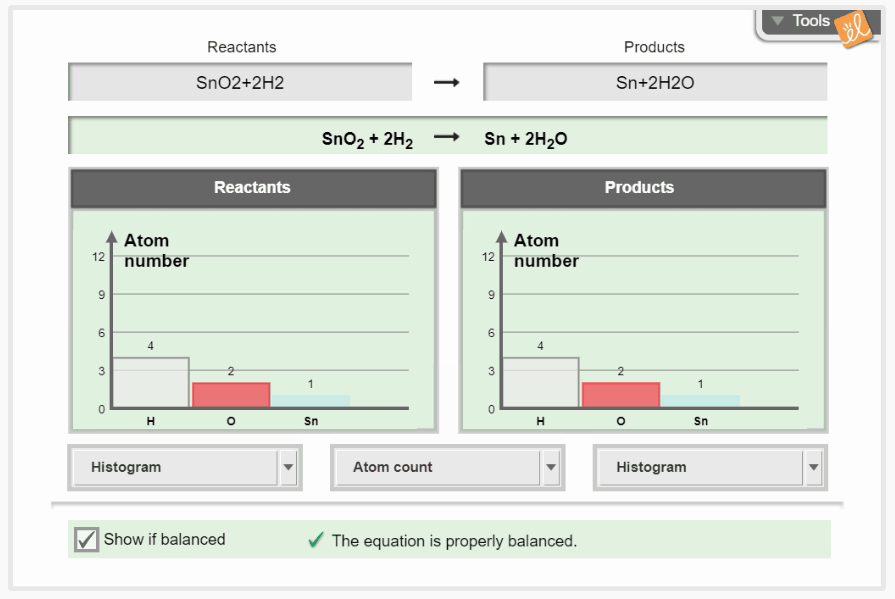
Practice balancing chemical equations by changing the coefficients of reactants and products. As the equation is manipulated, the amount of each element is shown as individual atoms, histograms, or numerically. Molar masses of reactants and products can also be calculated and balanced to demonstrate the conservation of mass.

Are you ready to make the periodic table come to life in your classroom? Your free trial is waiting.
Start My Trial