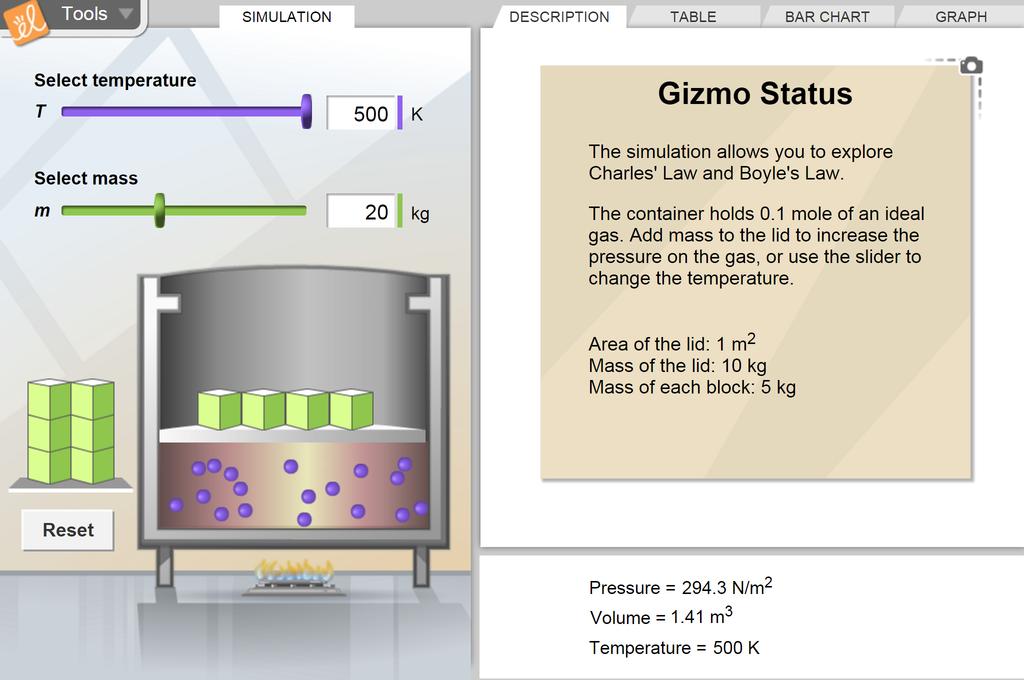
Boyle's Law and Charles's Law
Investigate the properties of an ideal gas by performing experiments in which the temperature is held constant (Boyle's Law), and others in which the pressure remains fixed (Charles's Law). The pressure is controlled through the placement of masses on the lid of the container, and temperature is controlled with an adjustable heat source. Gay-Lussac's law relating pressure to temperature can also be explored by keeping the volume constant.
Launch GizmoBoyle's Law and Charles's Law
Investigate the properties of an ideal gas by performing experiments in which the temperature is held constant (Boyle's Law), and others in which the pressure remains fixed (Charles's Law). The pressure is controlled through the placement of masses on the lid of the container, and temperature is controlled with an adjustable heat source. Gay-Lussac's law relating pressure to temperature can also be explored by keeping the volume constant.
5 Minute Preview
Use for 5 minutes a day.
Questions Recommend